Md. Hassan kajmir Mahmud (Industry Expert), Director, South West Composite Ltd. Gazipur, Bangladesh
Dr. Mohammad Jakir Hossain (Industry Expert), Divisional Research Officer, Forest Chemistry Department, Bangladesh Forest Research Institute (BFRI), Chittagong-4211
Md. Atiqur Rahman (Industry Supervisor), Assistant Manager (Utility), South West Composite Ltd. Gazipur.
Abstract
Last few decades, literature has been critically reviewed to inquire into current acetic acid manufacturing and purification process with their current feedstock to ensure sustainable business. Literature also discloses that rising global demand is accomplished by conventional manufacturing processes (chemical synthesis routes & fermentative routes) whereas multiple steps of operations are involved in purification processes such as distillation, evaporation, absorption and filtration consumed higher energy and manpower that is why to fail to ensure the sustainability. However, the adoption of membrane base separation techniques can easily resolve the distillation process within limited investment cost. But the leading raw materials methanol prices are gradually increasing for its versatile application. In the pursuit of sustainable development, the modern world introduces a novel renewable feedstock known as lignocelluloses biomass that has a great opportunity to convert directly to organic acids by several catalysis processes which minimize the methanol production and purification process. This paper prescribes future research towards the development of a green production pathway of cellulosic organic acids.
Keywords: Acetic acid; Sustainable business; Membrane base technology; Cellulosic biomass; Cellulosic organic acid.
Introduction
Acetic acid is the second simplest carboxylic acid after formic acid, traditionally used as a platform chemical to produce vinyl acetate, cellulose acetate, acetic anhydride whose derivatives are raw materials for the manufacture of adhesives, coating, cellulose plastics, aspirin, filament yarn, textile finishes, cement additives, packaging film and laminated safety glass for automotive and architectural application. In the textile fiber industry, acetic acid is a process solvent to produce purified terephthalic acid (PTA) from which polyester fibers are made. This important commodity is also used in the food industry as a flavoring agent and food preservative known as vinegar (4-6% diluted acetic acid) and in the pharmaceutical industry use as an antimicrobial agent. Glacial acetic acid is an excellent polar protic solvent that is frequently used as a solvent for recrystallization to purify organic compounds. The Global Acetic Acid Market size is forecast to reach USD 21.65 Billion by 2027 from USD 13.25 Billion in 2019, growing at a CAGR of 6.20% where Vinyl Acetate Monomer (VAM) application dominated the acetic acid market with a share of 35% in 2019 according to a new report published by “REPORTS AND DATA”.

In modern times, chemical synthesis routes and fermentative routes are the major production pathways to fulfill the global demand for acetic acid where Celanese, BP Chemicals, BASF, China Petrochemical, Dow chemical, Millennium Chemicals, Monsanto, Sterling Chemicals are the world’s biggest players. Through the chemical synthesis route methanol carbonylation process (Monsanto & Cativa), oxidation of aldehyde, hydrocarbon oxidation and syngas synthesis are key processes. On the other hand, the submerged fermentation-based Orleans method and the Frings-acetates based German method are some developments towards green production regimes. But only a small portion of global demand can be met from such eco-friendly biological technologies.
The purification process of acetic acid involves multiple units of operation such as distillation, evaporation, absorption, filtration, crystallization and alkali neutralization which consumes maximum energy and higher manpower making the process more complex. However, membrane-based (microfiltration, nanofiltration & ultrafiltration) purification processes bring forth their capability of eco-friendly production with higher purity in a very simple plant configuration that drastically cut off the total capital investment of both production pathways.
This paper has reviewed critically the traditional production process of acetic acid and focused on the major drawbacks associated with those processes while the modern world presents a green feedstock with membrane-based novel technologies.
Market study
Ethanoic acid or acetic acid is an organic compound with the chemical formula CH3COOH majorly produced by methanol carbonylation reaction in liquid form, which is colorless, strong distinct and pungent smell. For the acidic property, rising demand of methane carboxylic acid for Vinyl Acetate Monomer (VAM), Acetate Esters, Acetate Anhydride, Terephthalic Acid (TA) from various ends industries such as (Textiles & Adhesives, Coating & Painting, Plastics & Rubber) worldwide a major factor driving the growth rate of Acetic acid market. However, the market was negatively impacted by COVID-19 in 2020. A leading research company named “IMERC” indicated that the global acetic acid market was worth US$ 9.8 Billion in 2020. Keeping in mind the uncertainties of COVID-19, IMARC group experts are looking forwards, the market expanded at a CAGR of 6.80% to reach the market value of US$ 15.10 Billion during the forecasted period 2021-2026. On the other hand, the report produced by “BEROE Advanced Procurement” estimated the global capacity in 2019 was 18 MMT where global demand is 15.5 MMT. They also reported about the major supply market and share where the Asia Pacific region dominated the market and accounted for 70% and North America and European Union contributed 16% and 6% respectively. Globally Asian countries consumed 65% of total acetic acid whereas China is in the leading position producing 57% and consumed 52% of global acetic acid. According to the Bangladesh Bank report, in the 2018-19 and 2019-20 financial years Bangladesh imported approximately 20.89 Kilotons and 26.93 Kilotons respectively. The two biggest producers of virgin acetic acid are Celanese and BP Chemicals. Other major producers include Millennium Chemicals, Sterling Chemicals, Samsung, Eastman and Svensk Etanolkemi.
Current production processes of acetic acid
Acetic acid is predominately produced via a chemical synthesis route (88.80%) that involves homogeneous as well as heterogeneous catalytic methods. Methanol could be carbonylated to acetic acid through Monsanto process is the most adopted route, which further resolved as Cavita process with a choice of Iridium containing catalysts by BP Chemicals. Last few decades, the fermentative approach (11.20%) has also gained attention for the antifungal and antimicrobial properties of acetic acid. In the pursuit of sustainable development, an urgent paradigm shift is required in the acetic acid production process based on the raw material source instead of methanol, to develop and pursue more sustainable routes to reduce environmental burden. An approach is under construction with the development of transition metal oxides Nano-catalysts for the conversion of crystalline Nano cellulose (CNN) to Acetic acid which offers a green feedstock with an eco-friendly production process.
3.1 Chemical Conversion Process
The 99.8% pure acetic acid, sold in the name of glacial acetic acid can be manufactured by various processes such as methanol carbonylation, acetaldehyde oxidation and hydrocarbon oxidation. Acetic acid is also often used as a side product during the synthesis process of acrylic acid in heterogeneous catalytic conditions.
3.1.1 Methanol Carbonylation Process.
The most employed commercial route for the synthesis of acetic acid is the carbonylation of methanol also known as the Monsanto process which is followed for the production of 75% global acetic acid. By this process, Methanol and carbon monoxide are reacted in liquid phase in the presence of rhodium (Rh)- based catalyst at 150-200°C temperature and 30-50 bar pressure to produced acetic acid with 95% selectivity and 5% by-products like formic acid, formaldehyde, methanol, CO2 & H2. Hydrogen iodide is used as an alkali promoter in this process. The conversion rate of methanol to acetic acid in the Monsanto process depends on the concentration of water. The process has evolved with time and different strategies have been adopted to separate pure acetic acid from a mixture of water and by-products.
This process was modified by BP chemicals replacing rhodium-based catalyst [Rh(CO)2I2]¯ with iridium (Ir) catalyst [Ir(CO)2I2]¯ known as the Cavita process. The choice of iridium in the catalyzed process is greener and more efficient and has largely supplanted the Monsanto process, often in the same production plants. Catalytic amounts of water are used in both processes, but the Cativa process requires less, so the water-gas shift is suppressed and generated fewer by-products.

3.1.2 Acetaldehyde Oxidation
Before the commercialization of the Monsanto process, the oxidation of acetaldehyde was a prevalent process for the production of acetic acid. First, acetaldehyde is prepared by oxidation of ethylene via the Hoechst-Wacker process where PdCl2-CuCl2 is used as a catalyst. Hereafter, liquid phase acetaldehyde is oxidized to form 95% acetic acid and 5% side products like formic acid, formaldehyde, ethyl acetate in the presence of oxygen and manganese acetate catalyst.
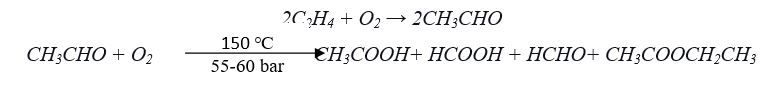
A single-step process is developed by the Japanese company “Showa Denko” for the conversion of ethylene to acetic acid using Lead and lead-platinum-based catalyst at high pressure with the selectivity of 87% acetic acid and valuable side products.
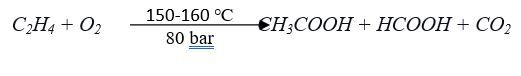
3.1.3 Hydrocarbon (butane, naphtha) oxidation process:
Hydrocarbons derived from petroleum stock such as butane and naphtha are utilized to generate acetic acid using cobalt acetate and manganese acetate catalyst. In consideration of using hydrocarbons where a great number of carbon atoms as raw materials, not only acetic acid but also acetone, formic acid, propionic acid and other byproducts are generated. However, the yield of acetic acid (50%) is comparatively lower than other processes but this process is more suitable for manufacturing a mixture of carboxylic acid. The reaction proceeds at a comparatively higher temperature range (150–230 °C) and pressure (50–60 bar).
3.1.4 Synthesis gas route to acetic acid:
An efficient integrated synthesis gas and methanol synthesis plant and acetic acid plant are available by a combination of current technology at the natural gas source. Haldor Topsoe proposed an integrated process that includes synthesis of methanol and dimethyl ether (DME) from syngas via a mixture of a catalyst such as (Cu-Zn-Al oxide) and dehydration catalyst (H-ZSM-5) in a first catalytic reaction condition approximately 220 ℃ and 40 bar pressure.
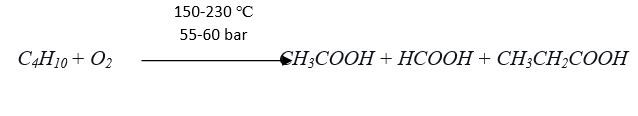
Similar to the Monsanto process, carbonylation of DME and methanol to acetic acid is carried out by the rhodium carbonyl complex catalyst with carbon monoxide being supplied from the synthesis gas process unit with the optimum reaction conditions of 170–250 ℃ and pressure 25–50 bar, where up to 95% acetic acid selectivity.
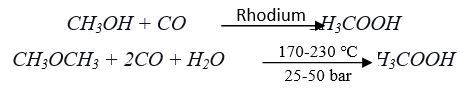
3.2 Fermentation route
Fermentation is a metabolic process by which renewable carbon resources are converted to acids, gases, or alcohol in aerobic or anaerobic conditions. The fermentative route is mostly adopted by the medical and food industry for the generation of hygienic and food-grade acetic acid concentrated at 6-10% which is known as vinegar. This process mainly involves the use of renewable carbon resources such as apple, grape, pears, honey, cane, coconut, date, syrup, cereals, hydrolyzed starch, beer and wine (white, red, sherry etc.). The fermentation process is mainly divided into two steps where yeast converts sugar into ethanol in anaerobic conditions then ethanol is oxidized to acetic acid aerobically by acetic acid bacteria (AAB). Acetobacterand Gluconacetobacter are the most used species among ten classified genera. Acetobacter pasteurianus is traditionally used for commercial production of vinegar with a concentration not exceeding 6% (v/v), whereas, Gluconacetobacter europaeus is utilized to produce high-concentration vinegar (10% v/v). The price of the flavoring vinegar varies with the source of raw materials and the region where it is generated.
There are mainly three methods of acetic acid production through fermentative routes such as Orleans method, Generator and the continuous submerged method.
3.2.1 Orleans Method
A well-established slow method of acidifying 5% wine to lower concentrated acetic acid is known as the Orleans process which has been used in France since 1670. This method is followed to prepare exotic brands of vinegar in different regions of the world with specific raw materials available in the specific season. In this process, wooden barrels are used to ferment the feed which is filled 75% total volume with alcohol fermenting liquid. The mother of vinegar is a gooey film that appears on the top of the alcohol products that contain the highest concentration of Acetobacters. The liquid is fermented for 1to 3 months at room temperature, approximately 850F (29 ℃).
3.2.2 Generator Method
Generator method or trickling methods was developed by a German chemist Schutzenbach in 1832, to overcome the slow rate of acidifying in the Orleans process by improving the Acetic Acid Bacteria (AAB) and substrate interaction. According to this process, the bacteria were grown and formed a thick slime coating around a non-compacting material like beechwood shavings, grape pulp, charcoal, or coke. Re-circulated fermenting liquid or mash trickles over the packing material toward the bottom while air moves from the bottom through inlets toward the top. The conversion rate of acidification is dependent upon oxygen concentration. A limited air supply means limited acetic acid production and lower generator temperatures while an overabundant air supply creates over-production and higher generator temperatures. The process takes about 3 to 7 days with the optimum temperature range 85 to 90 0F (29.6 to 32.2℃) where acidification started at temperature 70 0F (21.1 ℃). A temperature control system is necessary to prevent overheating and consequent inactivation of the bacteria.
3.2.3 Submerge Method:
Nowadays, the most common, faster and more efficient acetic acid fermentation process is submerged culture, which improves the general fermentation conditions such as aeration, stirring, heating, etc. Production plants are filled with large stainless steel tanks called acetators. The alimentator is filled with centrifugal pumps in the bottom that pump air bubbles into the tank, in much the same way that an aquarium pump does. As the pump stirs alcohol, Acetozyme nutrients are piped into the tank. The nutrients spur the growth of Acetobacters on oxygen bubbles. The fermenters are usually fitted with a heat exchanger for the maintenance of the optimum temperature during the fermentation process. The optimum temperature is (80-100 0F) for industrial production of 13% acetic acid. This fermentation process is very economical, of simple design with easy process control.
Limitation of the traditional production process of acetic acid:
Conventional acetic acid production plants are robust whereas multiple units of operations are executed with limited flexibility. Prominent and exclusive materials of construction are pre-required to build reactors that can resist high temperature and pressure while carrying out multiphase chemical reactions. More expensive feedstock and catalysts are used in chemical synthesis a route that is why the perfect selection of feedstock and recycling of catalysts become essential. But the contemporary world, representing an attractive feedstock known as cellulosic biomass, has a great opportunity to convert bio-chemical directly. Current production processes involve several steps like raw materials purification, catalysis, oxidation, distillation, condensation and crystallization, which increase the production cost. The operating conditions are harsh considering the process temperature and pressure along with the corrosive nature of acetic acid. Separation and Purification of the synthetic mixture is a complex, expensive and energy-consuming alarming process for its several distillations, absorption and drying actions. Discharge and dumping of waste acids or by-products (harsh chemicals) such as propanoic acid, butyric acid, butanone, ethyl acetate, formic acid and dichloro acetic acid in an open environment result in environmental pollution. Compared with the chemical synthesis process, the fermentation process for acetic acid is economically feasible with comparatively simple operations but the application is very limited for its slow and time-consuming process.
So, a novel, eco-friendly and the business viable green process is urgently required to develop from which the best utilization of green feedstock.
Membrane-based Purification Technologies:
A simple designed easy operating process membrane-based technologies can offer the separation of liquid, vapor and gas selectivity with a high degree of purity. Different types of processes are reported based on the pore size of the membrane such as microfiltration, ultra-filtration, and nano-filtration are used as a selective barrier for the separation of targeted molecules at different mass transfer rates. The simplicity of plant configuration mysteriously minimizes the capital investment. Microbial cells, having higher molecular weight and sizes can easily separate by a microfiltration membrane where the pore size (between 50 nm and 5 µm) and required pressure 1 to 4 bar. Ultra-filtration membranes have a pore size between 2nm and 50 nm and a pump able to reach 5 to 9 bar is required to separate cells, proteins and fats. In both microfiltration and ultra-filtration, the separation is based on size exclusion or sieving mechanism. Base on Donnan exclusion mechanisms nano-filtration membrane is developed whereas the average pore size is 1 nm and required a pressure (10-20 Bar) pump to create pressure and circulate the fluid. Nano-filtration is a newly developed technology that is mostly practiced for the separation of tiny neutral impurities and charged particles (microbial cells, proteins, unconverted sugar, salts, metal ions and the additional supplementary nutrients) present in aqueous solutions while increasing the final product purity in the permeate stream. Reverse osmosis (RO) membranes are usually non-porous and a high-pressure pump generating trans-membrane pressure above 20 bar is necessary. The deposition of coarse-sized particles over the membrane surface created fouling problems in membranes technologies. However, flat sheet cross-flow membrane modules can be practically minimized the fouling problem.
Globally Demanded a Novel Sustainable Process:
In recent decades concerns about the depletion of fossil fuel reserves and global warming caused by anthropogenic CO2 emissions have spurred the search for renewable chemicals and fuel sources. Cellulose is the most abundant renewable carbon resource on the planet and has great potential to be used as an alternative feedstock for the production of bio-based valuable platform molecules. Even though many processes and technological developments are reported recently, they fail to ensure the principle of “Green Chemistry” and the ever-growing market demand forces them to follow the current acetic acid synthesis process. The purification of acetic acid involves distillation, evaporation, absorption, filtration, crystallization and alkali neutralization operations, the key issue to overcome the economical, environmental and energy consumption barriers. However, fermentation is an eco-friendly process but cannot match the scale of current demand. A novel technological approach is required to develop a sustainable feedstock for the production of acetic acid. Cellulosic biomass emerges as a very promising source of raw material because of its larger number of carbon atoms originating from the fixation of CO2 by photosynthesis and, unlike starch or corn, cellulosic biomass is non-edible.
Lignocelluloses Biomass as a Renewable Feedstock:
Lignocellulose is a complex carbohydrate polymer, containing polysaccharides built from sugar monomers (xylose and glucose) and lignin, a highly aromatic material. So, Biomass is the most abundant renewable carbon resource on the planet and has great potential to be used as an alternative feedstock for the production of bio-based valuable platform molecules. But the conversion of biomass to organic chemicals is not an easy task because of biomass recalcitrance. After delignification, several processes are involved both in the chemical synthesis route and fermentation route of acetic acid production from biomass. In the fermentation process, the delignified biomass known as holo-cellulose (hemicelluloses & cellulose) is hydrolyzed with several enzymatic processes and then fermentation is done in aerobic or anaerobic conditions. In the chemical synthesis process, Alfa cellulose is isolated from holo-cellulose following several procedures of the pulp and paper industry. Dissolving cellulose or crystalline nano-cellulose (CNC) produced from Alfa cellulose or Micro-crystalline cellulose (MCC) via acid hydrolysis process. Then CNCs are catalyzed by several metal oxides for organic chemicals with sufficient conditions of time, temperature, pressure and concentration. The utilization of biomass can offer a sustainable alternative to produce acetic acid.
My Project Works:
From environmental concern, a project work named “Preparation of Acetic Acid from Sugarcane Bagasse Using Transition Metal oxide Nano-catalyst” is under construction under the supervision of Dr. Mohammad Jakir Hossain in Bangladesh Forest Research Institute, Chittagong.
Our collected samples are prepared by washing (cold & hot) and crushed in Lab Willey Mill Machine. Then powdered samples are treated with solvent, salt and alkali to remove extractives, lignin and hemicelluloses respectively following TAPPI methods. Some analytical experiments (such as FT-IR spectroscopy, X-ray diffraction & SAM analysis) ensure the Alfa cellulose crystal size and crystallinity index. We can successfully produce crystalline nano-celluloses (CNCs) or dissolving cellulose following the acid hydrolysis process. After that, we are trying to convert the glucose molecules to form organic acid (like acetic acid, oxalic acid & formic acid) by using metal (Fe, Cu, Zn, Mn, Ni) oxide nano-catalysts. The supported & unsupported catalysts are produced successfully to compare the catalytic activity.
Already we have done some catalysis reactions in alkaline conditions whereas the PH of the solution is put down after reaction. But unfortunately, for the Covid-19 pandemic situation, we cannot utilize the GC-MS facility to analyze reactions output.
Conclusion
The compound average growth rate of the acetic acid market is 5.5-6.2% per annum from a significant and large base capacity. Carbonylation of methanol by the Cativa process accomplishes a large portion of global market demand. Though methanol is a bio-based feedstock, this catalysis process required higher energy and manpower with multiple purification processes. The membrane-based nanotechnology improved the overall separation process. On the other hand, the fermentation route is commercially unsuitable for its long-run process to fulfill the global demand. However, the fermentation process is globally followed to produce food-grade high-quality acetic acid generally known as vinegar. For the growing acetic acid market, sustainable biochemical processes are in demand to develop recently. The best utilization of lignocelluloses biomass may offer alternative sustainable feedstock to develop an innovative commercial process for acetic acid production.
Corresponding Author: Dr. Mohammad Jakir Hossain, Divisional Research Officer, Forest Chemistry Department, Bangladesh Forest Research Institute (BFRI), Chittagong-4211.
Contact Number: (+88)1711-782885, 031681587. E-mail: smjakir080@gmail.com
Acknowledgement: The author’s gratitude to the Bangladesh Textile Today and South West Composite Ltd. Gazipur, Bangladesh, for their financial support.